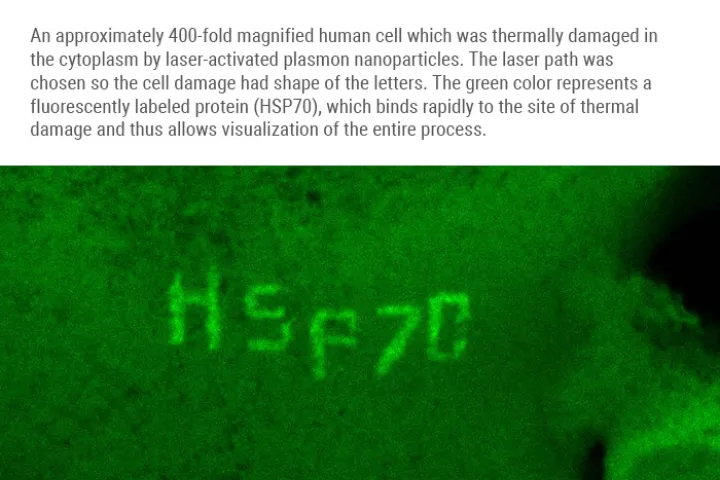
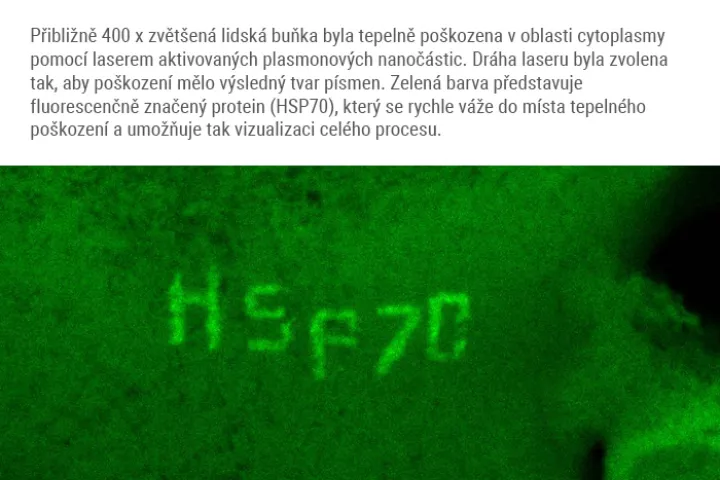
Olomouc 1.2. 2021
Proteins are the basic building blocks of living organisms. They arise in cells in a strictly controlled way during the translation process while information from individual genes is read, transcribed, and translated into a long chain of amino acids. The result is protein structures of various properties from an oxygen-binding blood dye to a tight rhino horn for example. However, proteins are not eternal. They have only a temporary lifespan and the cell may not always be able to produce them correctly. Old, damaged, and defective proteins made during production can be a problem and even life-threatening in extreme cases. If the cell cannot get rid of such proteins, or at least neutralize their harmful effect, diseases so-called proteinopathies develop. An example is the accumulation of beta-amyloidosis, TAU, and other problematic proteins causing cellular stress and total tissue degeneration. It can grow into Alzheimer's or Parkinson's disease, or other degenerative diseases including cancer.
The study of protein stress at the cellular level is an important part of the research of both diseases. Researchers are studying the factors that cause or exacerbate this stress which have potential use in the treatment of cancer. Scientists are also interested in how a cell handles defective proteins or how to pharmacologically affect these processes. However, there is still a limited amount of current scientific possibilities available to target damaged proteins in the cell. In contrast, DNA can be damaged very precisely in the cell. We can literally monitor how the cell’s repair mechanisms cope with the damage in real time. Until now, this has not been practically possible to do with proteins. Scientists had no choice but to expose the cells in bulk to elevated temperatures or various toxins, and then measure the resulting effects on the entire cell population using indirect biochemical methods.
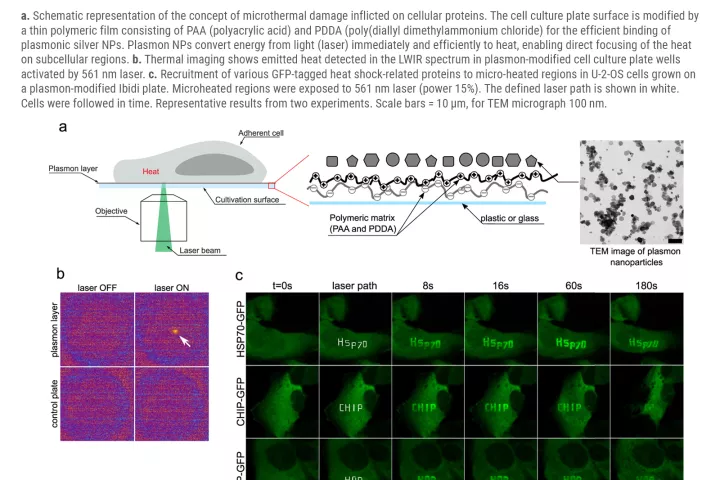
A team of scientists from the Institute of Molecular and Translational Medicine Medical in Olomouc (IMTM) in cooperation with the Faculty of Science and its science centre RCPTM (both Palacký University), Masaryk Memorial Cancer Institute in Brno, and with the infrastructure support of the Czech BioImaging infrastructure, led by Dr. Martin Mistrík managed to develop a revolutionary method combining targeted damage to proteins within individual cells and simultaneous microscopic analysis. The method uses special silver nanoparticles, known as plasmonic nanoparticles, which function as miniature but with very powerful sources of heat that can be activated by a targeted laser beam.
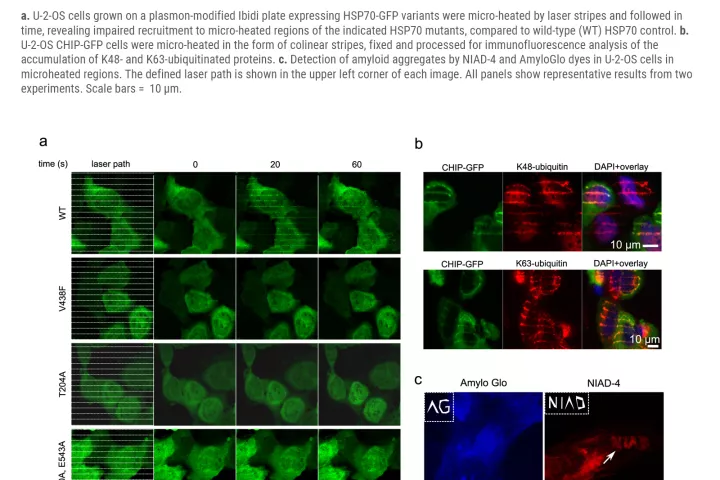
"After irradiation, plasmonic nanoparticles absorb laser light and subsequently emit intense heat, which on a microscopic scale damages proteins in the immediate vicinity,” says study co-author Aleš Panáček, who studies nanoparticles. Thermal damage mimics natural protein degeneration and triggers appropriate cellular processes. Thanks to the method, we can choose a specific part of the cell to which the laser will point, and literally burn the cell in the given area. Then, we can map the subsequent processes. In a study whose contribution to the scientific community was praised by the prestigious journal Nature Communications (in its Friday publication), the researchers presented way more than this method. „With its use, we also revealed unsuspected connections amid the ways in which a cell handles damaged proteins. We also discovered a new factor that significantly contributes to their removal. In the future, pharmacological targeting of such factors will help us to make antitumor thermotherapy more effective, or it will lead to the prevention or treatment of the degenerative diseases mentioned above ", explains the importance of this study its first author Martin Mistrík from IMTM.
The whole article in nature communications: HERE!
Peter VANEK
Institute of Molecular and Translational Medicine LF UP Hněvotínská 1333/5 779 00 Olomouc Email: peter.vanek@upol.cz Tel: 775 050 355